Online Encyclopedia
Atom
- For alternative meanings see atom (disambiguation).
| Atom | ||||||
|---|---|---|---|---|---|---|
|
||||||
| Classification | ||||||
|
||||||
| Properties | ||||||
|
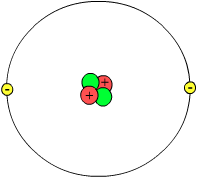
An atom is a microscopic structure found in all ordinary matter around us. Atoms are composed of 3 types of subatomic particles:
- electrons, which have a negative charge;
- protons, which have a positive charge; and
- neutrons, which have no charge.
Atoms are the fundamental building blocks of chemistry, and are conserved in chemical reactions. An atom is the smallest particle differentiable as a certain chemical element; when an atom of an element is divided, it ceases to be that element. Only 91 elements have been identified as occuring naturally on earth. Each element is unique by the number of protons in each atom of that element. Every atom has a number of electrons equal to its number of protons; if there is an imbalance, the atom is called an ion. Atoms of the same element can have different numbers of neutrons. Atoms with different numbers of neutrons are called isotopes of the chemical element.
Other elements have been artificially created, but they are usually unstable and spontaneously decay into natural chemical elements by nuclear fission.
Though only 91 unique types of atoms exist, atoms are able to bond into molecules. Molecules are made up of multiple atoms. A molecule of water is a combination of 2 hydrogen and one oxygen atoms.
Because of their ubiquitous nature, atoms have been an important field of study for many centuries. Current research focus on quantum effects, such as in Bose-Einstein condensate.
| Contents |
|
|
Atomic theory
Main article: Atomic theory
The atomic theory is a theory of the nature of matter. It states that all matter is composed of atoms.
Structure
Historical Theories
Democritus, a Greek philosopher in the #th century BC]], presented the first theory of atoms. He held that each atom had a different shape, like a pebble, the governed the atom's properties.
Since Democritus' time, many theories for the structure of the atom have been suggested:
Today, the widely accepted theory is the wave model.
The Wave Model
The wave model of the atom is based on the Bohr model, but takes into account recent developments and discoveries in quantum mechanics.
It states that:
- Atoms are composed of subatomic particles (protons, electrons, and neutrons).
- However, much of an atom's volume is empty space.
- At the center of the atom is a tiny, positively charged nucleus composed of protons and neutrons (known as nucleons).
- The nucleus is more than 100,000 times smaller than the atom. If an atom was expanded to the size of Heathrow Airport, the nucleus would be about the size of a golf ball.
- Most the atom's space is taken up by orbitals containing electrons in a certain electron configration .
- Each orbital can hold up to two electrons, and is governed by three quantum numbers: the principle , azimuthal, and magnetic.
- Each electron in an orbital has a unique value for the fourth quantum number, spin .
- Orbitals are not physical contructs, but are actually probability distributions of where two electrons having equal values for the first three quantum numbers might be.
- As electrons join an atom, they fall into the lowest energy shell; that is, the orbitals closest to the nucleus (the first shell). Only the electrons in the outermost orbital (the valence shell) are available for atomic bonding; see "Valence and bonding" for more information.
Elements and isotopes
Atoms are generally classified by their atomic number, which corresponds to the number of protons in the atom. The atomic number decides which family or element the atom belongs to. For example, carbon atoms are those atoms containing 6 protons. All atoms with the same atomic number share a wide variety of physical properties and exhibit the same chemical behavior. The various kinds of atoms are listed in the Periodic table. The mass number or nucleon number is the total of protons and neutrons. The number of neutrons does not have any effect on the element of the atom - within an element family are several members, each with the same atomic number but different mass numbers. These are called isotopes. To write the name of an isotope, we write the name of an element followed by its mass number, eg. Carbon 14 (which contains 6 protons and 8 neutrons in each atom).
The simplest atom is the hydrogen atom, having atomic number 1 and consisting of one proton and one electron. The hydrogen isotope containing 1 additional neutron is called deuterium; the hydrogen isotope with 2 additional neutrons is called tritium. It has been the subject of much interest in science, particularly in the early development of quantum theory.
Valence and bonding
The chemical behavior of atoms is largely due to interactions between electrons. Electrons of an atom must remain within certain, predictable electron configurations. Electrons fall into shells based on their relative distance from the nucleus (see Atomic structure for more details). The electrons in the outermost shell, called the valence electrons, have the greatest influence on chemical behavior. Core electrons (those not in the outer shell) play a role, but it is usually in terms of a secondary effect due to screening of the positive charge in the atomic nucleus.
Each shell, numbered from the inside, can hold up to a limited number of electrons due to its orbitals:
- Shell 1: 2 electrons
- Shell 2: 8 electrons
- Shell 3: 8 or 18 electrons (depending on the element)
Electrons fill orbitals from the inside out, beginning with shell one. Higher numbered shells only exist when made necessary by the number of electrons. Whichever shell is currently most outward is the valence shell, even if it only has one electron.
The number of electrons in an atom's valence shell governs it's bonding behavior. Therefore, elements with the same number of valence electrons are grouped toegether in the periodic table of the elements. Group (i.e. column) 1 elements contain one electron on their outer shell; Group 2, two electrons; Group 3, three electrons; etc. As a general rule, the fewer electrons in an atom's valence shell, the more reactive it is. Group 1 metals are therefore very reactive, with caesium, rubidium, and francium being the most reactive of all elements.
Every atom is much more stable (i.e. less energetic) with a full valence shell. This is achieved either by sharing electrons with neighboring atoms or by completely removing electrons from or to other atoms. This is known as chemical bonding and serves to build atoms into molecules. Five types of bonds exist:
- ionic bonds;
- covalent bonds;
- coordinate covalent bonds;
- hydrogen bonds; and
- metalic bond s.
Etymology
The word atom is derived from the Greek atomos, indivisible, from a-, not, and tomos, a cut. Till the 19th century many people believed atoms to be tiny hard spheres, like minute billiard balls. They thought they had no internal parts and could not be created, divided or destroyed.
See also
- Atomism
- Exotic atom
- Radioactive isotope
- List of particles
- Individual (same literal meaning)
- Infinite divisibility
- Chemical bond
External link
- The American Heritage Dictionary (contains popups)
|
Particles in Physics - Composite particles |
