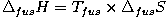The increase in entropy when melting a substance is called the Entropy of fusion. This is always positive since the degree of disorder increases in the transition from an organized crystalline solid to the disorganized structure of a liquid. It is denoted as ΔfusS and normally expressed in J / mol · K
A natural process such as a phase change will occur when the associated change in the Gibbs free energy is negative. It follows that the entropy of fusion is related to the melting point and the heat of fusion: